The growing weight of orphan oncology
Over the past decade, 67% of oncology drug approvals have carried orphan designation.
For partnership teams, this makes rare oncology one of the most capital-efficient entry points for first-in-class innovation in cancer treatment. For R&D teams, rare indications help prevent herding in the drug development pipeline – which now averages 9 assets per investigated oncology-related target.
What Explority AI does — and what “top therapies” means
Trained on all prior publications connected to drug discovery outcomes across 5,846 rare diseases, our LLMs forecast approval probability for early-stage drugs with precision that exceeds the pharmaceutical industry’s success rates.
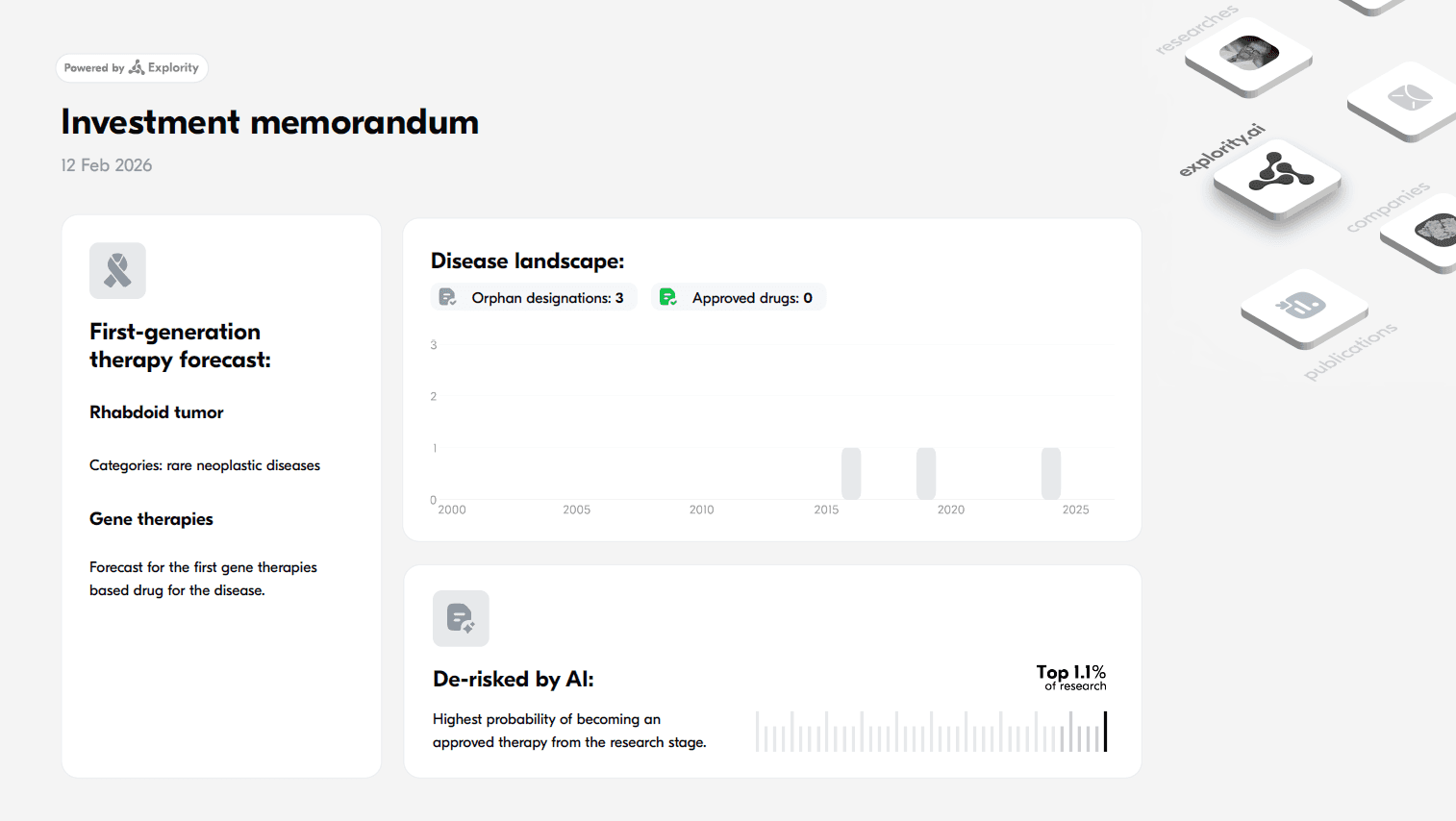
Below are investment reports generated by our platform, including AI-based percentile rankings that estimate each drug’s likelihood of success among new rare disease candidates.
Top 10 emerging first‑in‑class oncology drugs:
The first gene therapy for Malignant peripheral nerve sheath tumor
The first cell therapy for Malignant peripheral nerve sheath tumor
The first gene therapy for Familial hemophagocytic lymphohistiocytosis
Partner with Explority to turn information into impact. Whether you're planning your next phartership, selecting next R&D idea or just have questions—drop us a message. Let’s explore how we can work together to solve rare diseases.


